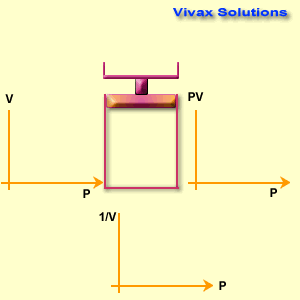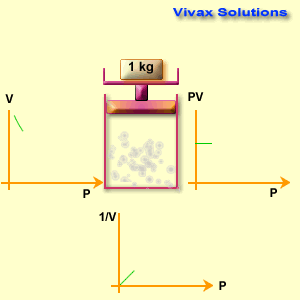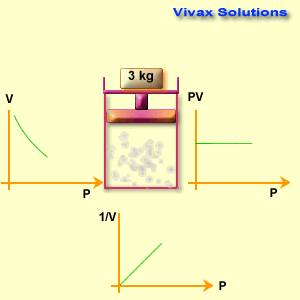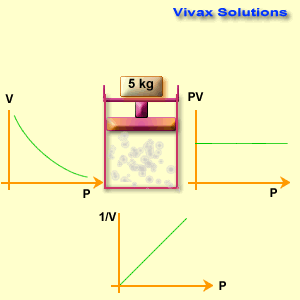
The volume of a fixed mass of gas is inversely proportional to its pressure at constant temperature.
p ∝ 1 / v
p = k 1 /v
pv = k
If the pressure and volume of a fixed mass of gas take the values of p1 v1and p2 v2 respectively,
p1 v1 = p2 v2
E.g.
The pressure of a fixed mass of air is 20 Pa and volume is 8 cm3. Its pressure is doubled while keeping the temperature constant. Find the volume.
p1 = 20, v1 = 8, p2 = 40
According to Boyle’s law,
p1v1 = p2v2
20 x 8 = v2 x 40
v2 = 4cm3
Leave a Reply