Shapes of molecules and VSEPR model
The shapes of molecules refer to the three-dimensional arrangements of atoms within a molecule. These shapes are determined by the arrangement of electron pairs around the central atom(s) and are crucial for understanding the molecule’s properties and behavior.
Molecules adopt specific shapes due to the arrangement of their electron pairs, which are governed by the principles of valence shell electron pair repulsion (VSEPR) theory. According to VSEPR theory, electron pairs in the valence shell of an atom repel each other and arrange themselves in a way that minimizes repulsion, resulting in predictable molecular geometries.
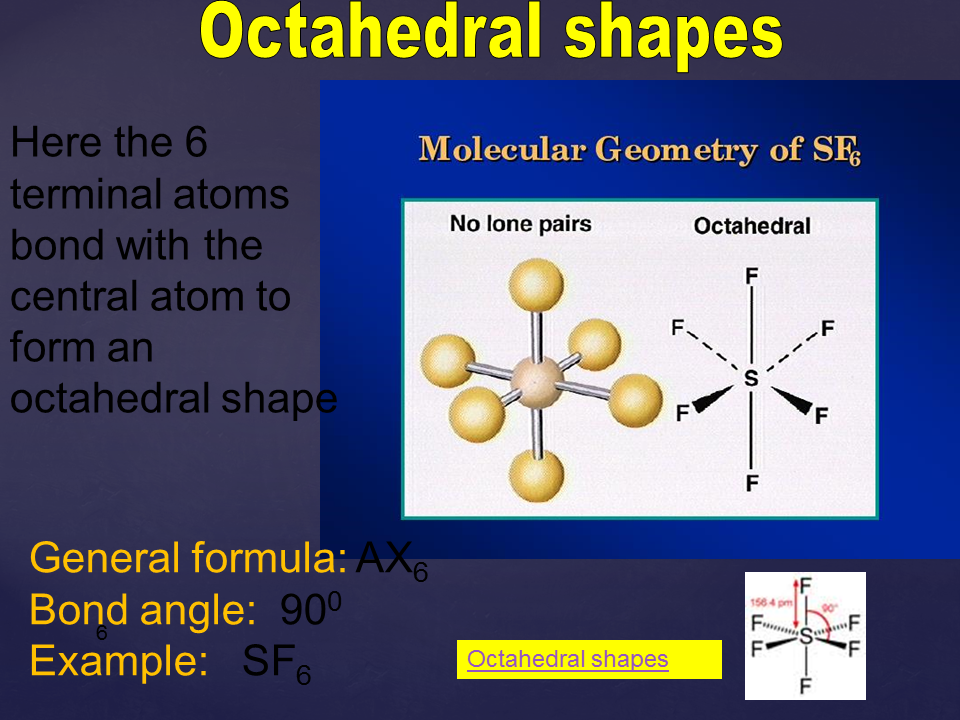
Common molecular shapes include linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral, among others. These shapes can influence various properties of molecules, such as polarity, bond angles, and reactivity.
Understanding the shapes of molecules is essential in fields such as chemistry, biochemistry, and materials science, as it provides insights into molecular interactions, chemical reactions, and the properties of substances.