The volume of a fixed mass of gas is inversely proportional to its pressure at constant temperature.
p ∝ 1 / v
p = k 1 /v
pv = k
If the pressure and volume of a fixed mass of gas take the values of p1 v1and p2 v2 respectively,
p1 v1 = p2 v2
E.g.
The pressure of a fixed mass of air is 20 Pa and volume is 8 cm3. Its pressure is doubled while keeping the temperature constant. Find the volume.
p1 = 20, v1 = 8, p2 = 40
According to Boyle’s law,
p1v1 = p2v2
20 x 8 = v2 x 40
v2 = 4cm3
Boyle’s Law Worksheet
1. Key Concept
Boyle’s Law: At constant temperature, the pressure of a gas is inversely proportional to its volume.
Formula:
P_1 V_1 = P_2 V_2
where
P_1 = initial pressure
V_1 = initial volume
P_2 = final pressure
V_2 = final volume
2. Quick Notes
If volume decreases, pressure increases (and vice versa).
Units must be consistent (e.g., L and L, kPa and kPa).
Temperature must stay constant.
3. Practice Problems
Q1. A gas has a volume of 3.0 L at a pressure of 200 kPa. What will its volume be at a pressure of 300 kPa?
Q2. A sample of nitrogen occupies 8.0 L at 100 kPa. If the pressure is decreased to 25 kPa, what is the new volume?
Q3. A gas is kept at a constant temperature. The pressure of the gas changes from 2.0 atm to 5.0 atm, and its final volume is 1.2 L. What was the initial volume?
Q4. Oxygen gas has a volume of 750 mL at 120 kPa. What pressure is needed to compress it to 500 mL?
Q5. A balloon has a volume of 2.5 L at 1.0 atm. If the balloon rises to where the pressure is 0.5 atm, what is its new volume?
4. Challenge / Extension
Sketch a graph of Pressure (y-axis) vs. Volume (x-axis). What is the shape of the curve?
Explain why the graph makes sense based on Boyle’s Law.
What is Boyle’s Law?
Boyle’s Law states that:
At constant temperature, the pressure of a fixed amount of gas is inversely proportional to its volume.
Mathematically:
P xV = {constant}
So, if the volume decreases, the pressure increases, and vice versa — provided temperature remains unchanged.
Boyle’s Law Experiment
Apparatus:
A sealed glass tube (J-tube or syringe setup)
Trapped gas (usually air)
Mercury or a piston to vary the pressure
Pressure gauge / scale to measure pressure
Scale to measure volume
Method (Mercury Tube Version):
A column of air is trapped in a closed end of a long glass tube.
Mercury is poured into the open end.
As mercury rises, it compresses the trapped air, reducing its volume.
The pressure on the trapped air equals the atmospheric pressure plus the mercury column height.
The corresponding volume of the air column is measured.
This is repeated for different mercury levels (different pressures).
Alternative (Modern Syringe Version):
A syringe with a sealed tip traps a fixed amount of air.
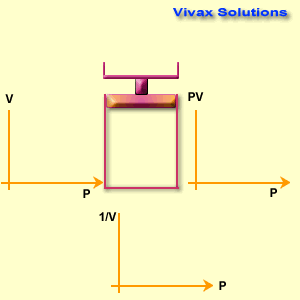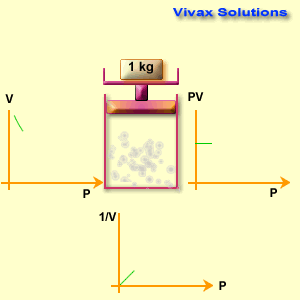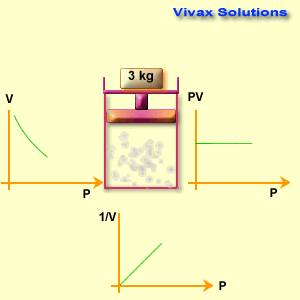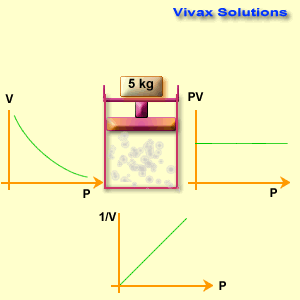
Different known weights are placed on the plunger to apply pressure.
The volume reading on the syringe is recorded for each pressure applied.
Observations
When pressure increases, the gas volume decreases.
When pressure decreases, the gas volume increases.
The product P \times V remains nearly constant for all readings (within experimental error).
Conclusion
The experiment confirms Boyle’s Law:
P =1/V{at constant temperature})
Graphically:
P vs V → a downward curve (hyperbola).
P vs 1/V → a straight line.
Leave a Reply