See on Scoop.it – PHYSICAL SCIENCES BREAK 1.0
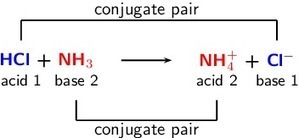
Definition 1: Acids
A Bronsted-Lowry acid is a substance that gives away protons (hydrogen cations H+), and is therefore called aproton donor.
Definition 2: Bases
A Bronsted-Lowry base is a substance that takes up protons (hydrogen cations H+), and is therefore called aproton acceptor.
Chipa Thomas Maimela‘s insight:
See on everythingscience.co.za
Leave a Reply