Atomic Structure.
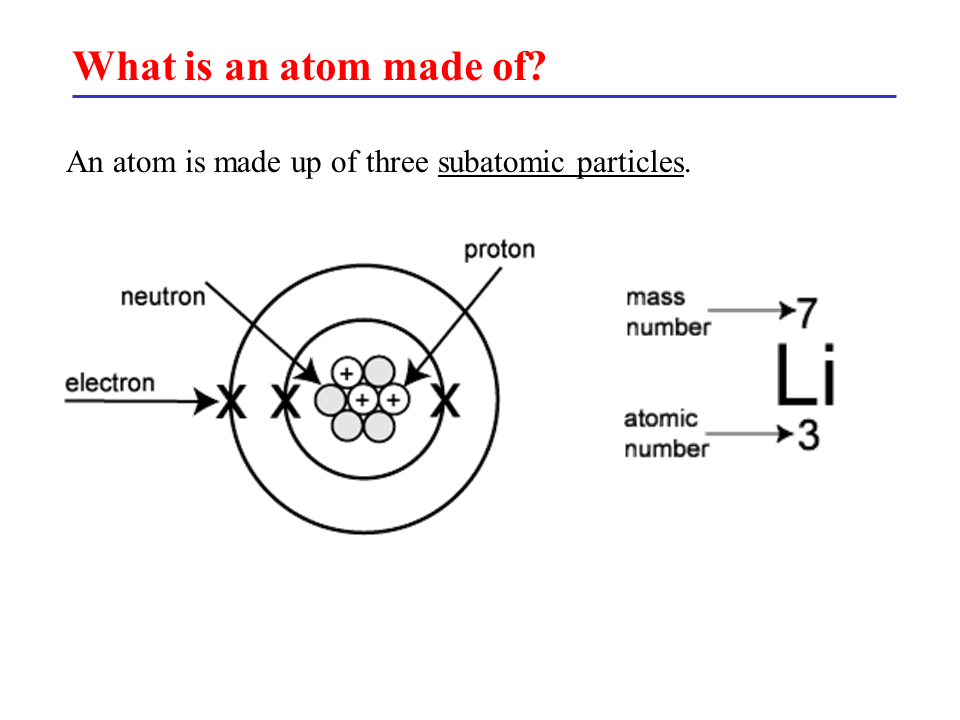
Atomic structure refers to the composition and arrangement of subatomic particles within an atom. Atoms are the basic building blocks of matter and consist of three primary subatomic particles: protons, neutrons, and electrons.
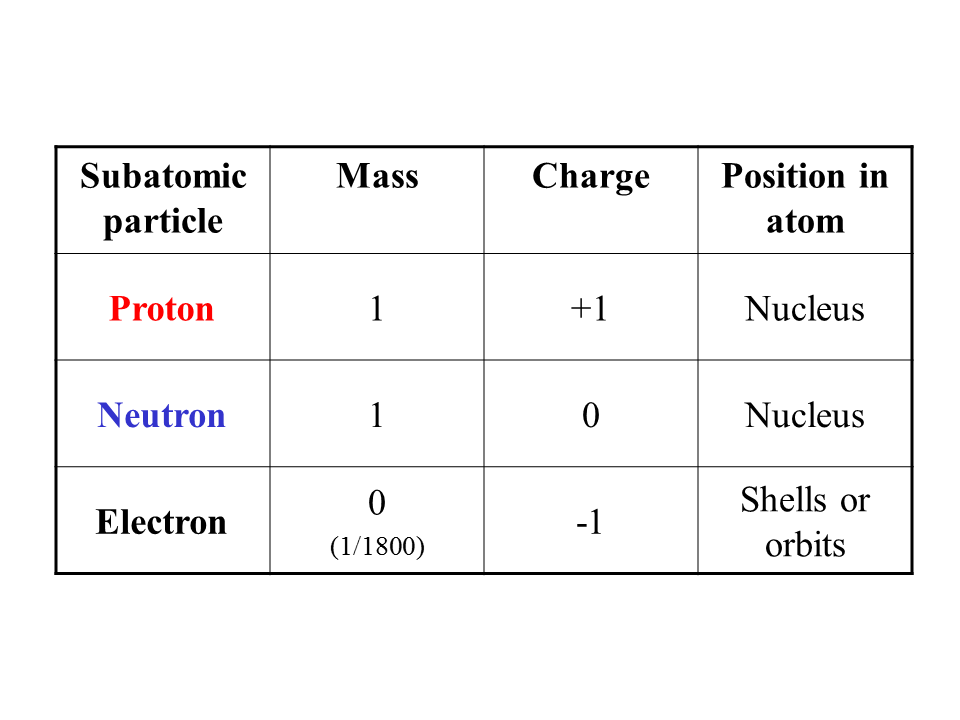
Protons: Protons are positively charged particles found in the nucleus of an atom. They have a relative mass of approximately 1 atomic mass unit (amu) and a charge of +1.
Neutrons: Neutrons are neutral particles, meaning they have no electric charge. Like protons, they are located in the nucleus of an atom. Neutrons also have a relative mass of approximately 1 amu.
Electrons: Electrons are negatively charged particles that orbit the nucleus of an atom in specific energy levels or shells. They have a much smaller mass compared to protons and neutrons, approximately 1/1836 amu.
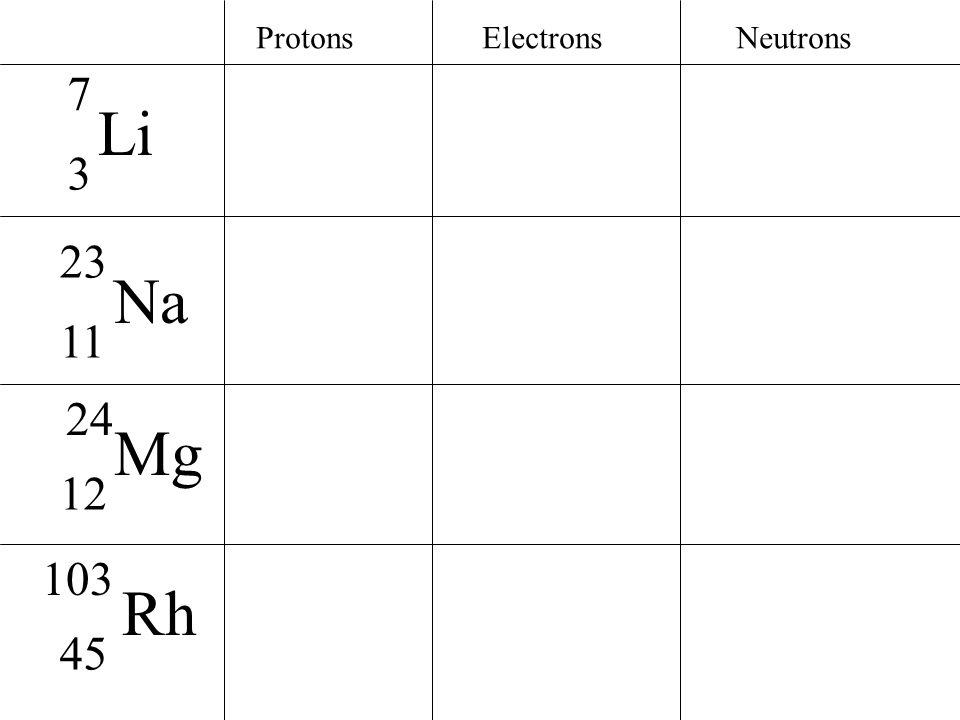
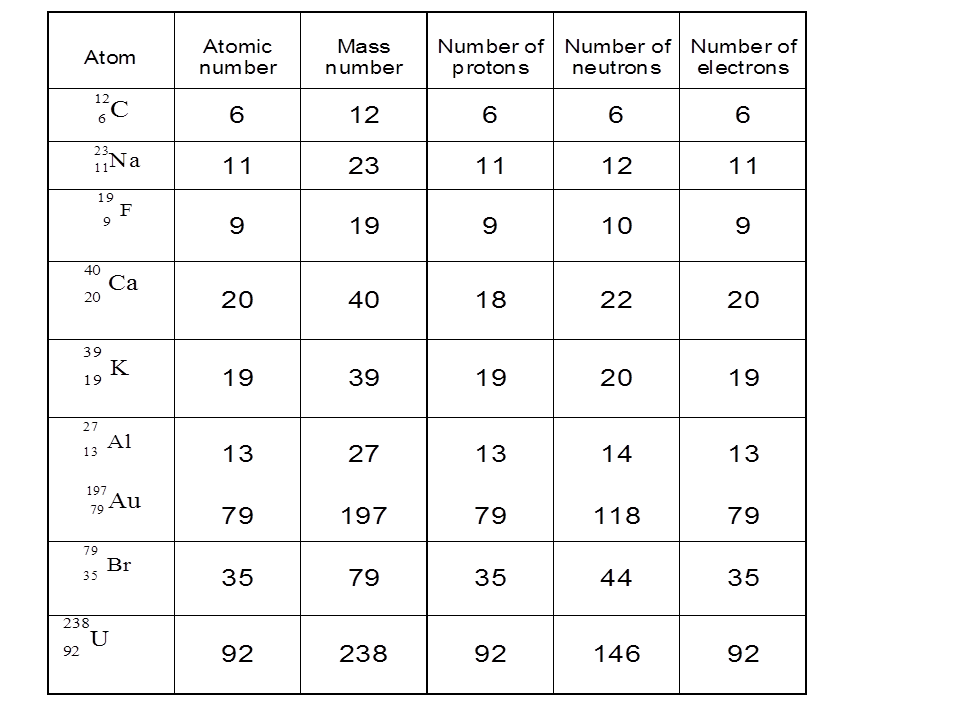
The number of protons in an atom determines its atomic number (Z) and identifies the element. For example, hydrogen has one proton, helium has two, and so forth. The sum of protons and neutrons in the nucleus gives the atom’s mass number (A).
The electrons are arranged in energy levels or shells surrounding the nucleus. These energy levels are quantized, meaning electrons can only occupy specific energy levels, and each shell can only hold a certain number of electrons. The innermost shell can hold up to 2 electrons, while subsequent shells can hold more. The arrangement of electrons in these energy levels follows specific rules, such as the Aufbau principle, Hund’s rule, and the Pauli exclusion principle.
GET A FREE TEXTBOOK FOLLOW THE LINK
ATOMIC STRUCTURE
TEST & MEMO
Gr-10-science-tests/